
Temperature - The moisture holding capacity of air increases with temperature.
Pressure and Temperatures - Air density at pressure ranging 1 to 10 000 bara (14.5 - 145000 psi) and constant selected temperatures.

Specific heat (heat capacity) and entropy unit converter Properties of air along the boiling and condensation curves - Imperial units:
DYNAMIC VISCOSITY AIR CURVE FITTING FULL
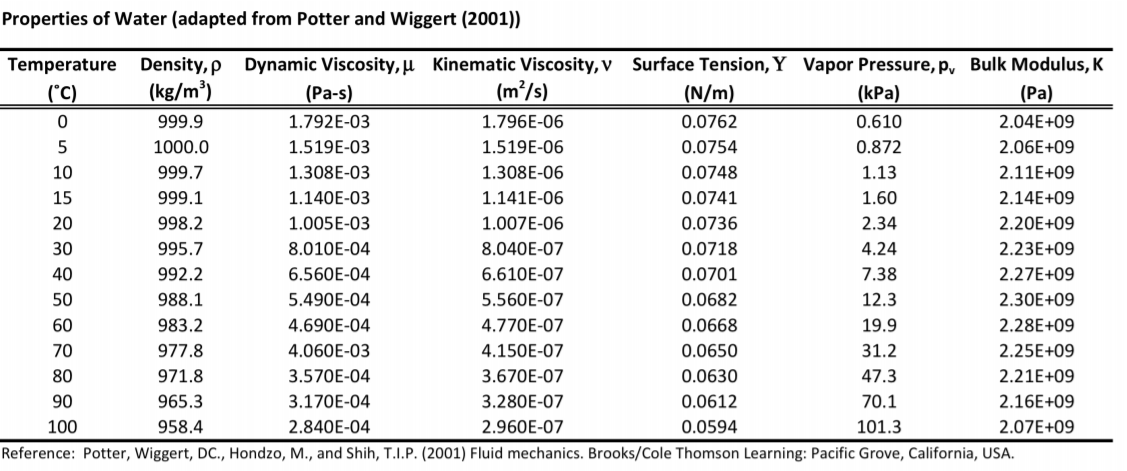
Properties of air along the boiling and condensation curves - SI units:įor full table with thermal conductivity, dynamic and kinematic viscosity - rotate the screen! Temperature The following figures show how the properties of air changes along the boiling and condensation curves (shown in the figure above) - SI units: See also properties of Air at varying temperature and pressure: Density and specific weight at varying temperature, Density at varying pressure, Diffusion Coefficients for Gases in Air, Prandtl Number, Specific heat at varying temperature and Specific heat at varying pressure, Thermal Conductivity, Thermal Diffusivity, and Air thermophysical properties at standard conditions and Composition and molecular weight, Tabulated values given in SI and imperial units, and unit converters are given below the figures. The figures below show the changes in thermophysical properties along the air boiling and condensation curves: Vapour pressure, density, viscosity, heat capacity, thermal conductivity. Triple point: The temperature and pressure at which the three phases (gas, liquid, and solid) of a substance coexist in thermodynamic equilibrium At the critical point, defined by the critical temperature T c and the critical pressure P c, phase boundaries vanish.Ĭritical temperature of air: 132.63 K = -140.52 ☌ = -220.94 ☏Ĭritical pressure of air: 37.363 atm = 37.858 bar = 3.7858 MPa (MN/m 2) = 549.08 psi (=lb f/in 2) At higher temperatures, the gas cannot be liquefied by pressure alone.

The curve between the triple point and the critical point shows the air boiling point with changes in pressure.Ĭritical point: The end point of the pressure-temperature curve that designates conditions under which a liquid and its vapor can coexist. The air phase diagram shows the phase behavior with changes in temperature and pressure. However, at low temperature and high pressures the gas mixture becomes a liquid. At standard conditions, air is a mixture of gases.


 0 kommentar(er)
0 kommentar(er)
